The First Law of Thermodynamics ?
The first law of thermodynamics, also known as the conservation of energy principle,
provides a basis for studying the relationships among the various forms of
energy and energy interactions.
The first law of thermodynamics states that energy can be neither created nor destroyed during a process, it can only change
forms. Therefore, every bit of energy should be accounted
for during a process. Implicit
in the first law statement is the conservation of energy.
The conservation of energy principle can be expressed as follows: The net
change in the total energy of the system during a
process is equal to the difference between the total energy entering and the total energy leaving the system during that process. That is,
Also, energy
can exist in numerous forms such as internal (sensible,
latent, chemical, and nuclear), kinetic, potential, electric,
and magnetic, and their sum
constitutes the total energy E of a system. In the absence of
electric, magnetic, and surface tension effects (for simple compressible systems), the change in the total energy of a system during a process is the sum of the changes in its internal, kinetic, and potential energies and can
be expressed as

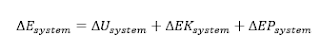

Comments
Post a Comment